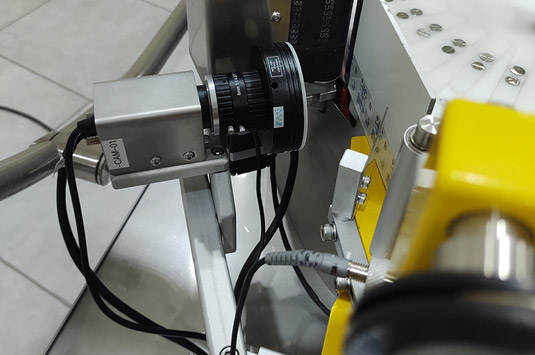
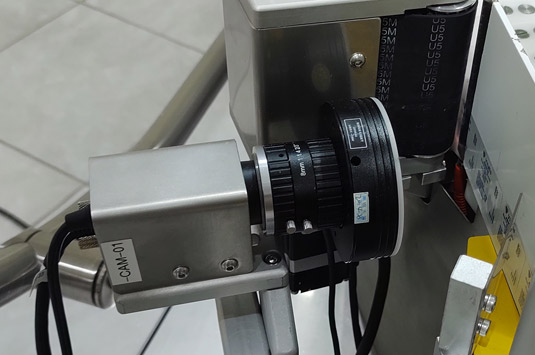
The equipment includes the codification and verification system ONELITE Print & Check.
Provided with a thermal ink transfer printer VideoJet (TTO) and an inspection camera which allows print and verify the OCR/OCV (Optical Character Recognized/Optical Character Verification) on labels, information as: batch, production date, expiration date or others before its application in the products.
The products to be labeled are positioned in loading trays on the entry conveyor. The products download, for both accepted and rejected are also done in accumulation trays. Ejection system with collection trays for rejected products.
Also offers an easy operation by the HMI control panel of 15” touchscreen, where the operator can coordinates the labeling, printing and verification system, obtaining a central database for the traceability of each product (optional). It allows too save programs with the specific configuration of every product to be produced. Has different access levels with users and password required, event log and production reports. The ONELITE system is in compliance with CFR 21 part 11 (FDA) and GAMP5 (ISPE).
PhC Cosmetics / Pharma Line: VLM-15000
Labelling machine for vials with print and check system VLM-15000
Automatic labeling machine to applicate self-adhesive labels on cylindrical products, vials or ampoules in vertical position. This labels could be printed and verified on the same equipment, with an eject module for non-compliant inspected units.
Details Gallery
Integrated Print and Check System
Mechanical adjustments with position indicators
Integrated Print and Check System
Outstanding Features
- Provides complete coordination between printing, labeling and rejection system.
- Simple adjustment for placing the labeling head for a variety of vial sizes.
- Automatic synchronization of speed for all systems from Panel PC.
- Rejection station for labeled vials with misinformation.
- USB and Ethernet ports for transferring data with other equipment and downloading the Event Log.
- Labelling in vertical position system.
- Loading and downloading of products in storage trays.
- Adequate safety system that guarantees reliable operation of the equipment, preserving the safety of the operator.
- USB and Ethernet ports for data transference and event log downloading.
- Anti-accident structure of stainless steel and 6 mm polycarbonate that allows excellent visibility towards the inside of the machine.
- Minimum space required.
- Quality control of computerized system according to guidelines given by GAMP 5 and 21 CFR Part 11 (FDA).
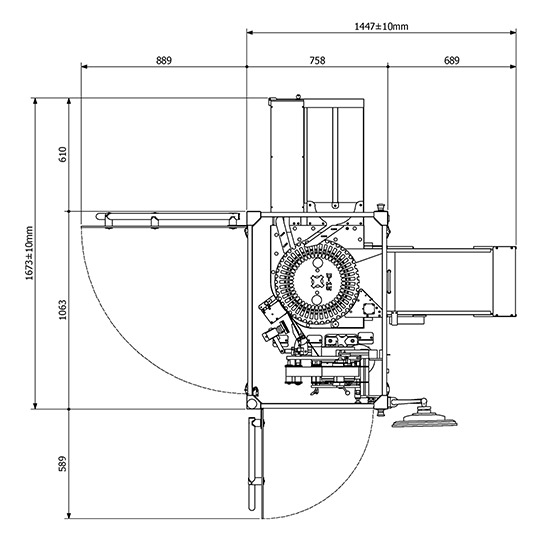
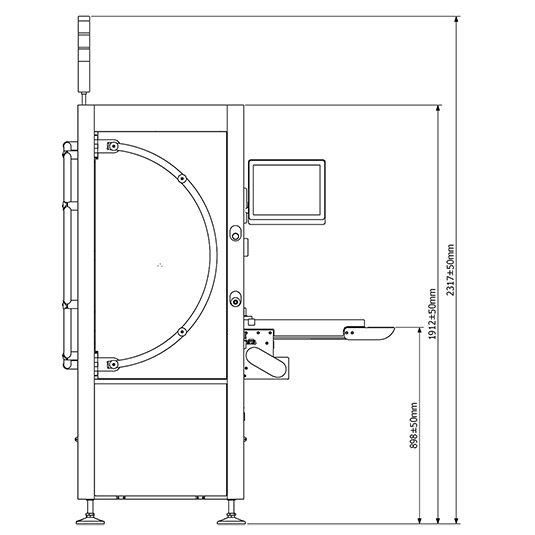
Actual Dimensions
Upper Layout
Front Layout
Technical Data
| Dimensions (mm) - Lenght x Width x Height | 1450 x 1670 x 2345 |
| Weight (kg) | 350 |
| Installed Power(KVA) | 2 |
| Production Capacity (vials / min) * | 250 |
| Admisible products (mm) - Diameter x Height | 10.75 / 22 (20 ml) x 30-112 ** |
| Admisible labels (mm) - Height x Width | 15-60 x 20-50 |
| Ribbon width (mm) | 32 |
| Printer | Thermal Transfer Overprinting (TTO). |
| Machine vision camera | High resolution |
| * Maximum output may vary depending on product size. ** Other diameters and heights on request. May require change of center disc. |
|
The mentioned characteristics can be modified without prior notice if the Company considers it for manufacturing or operating reasons. Operation voltages can be adaptable to each country or working environment.
Aware of the need to maximize space in the clean rooms in pharmaceutical industry, the size of our machines is one of the main premises at the time of the design of our entire Pharma Line.
GET IN TOUCH! Take advantage of the knowledge of our Sales Team by calling us today on +54 260 442 1956 or from here . We will be happy to help you to come up with the most effective solution for your requirements.